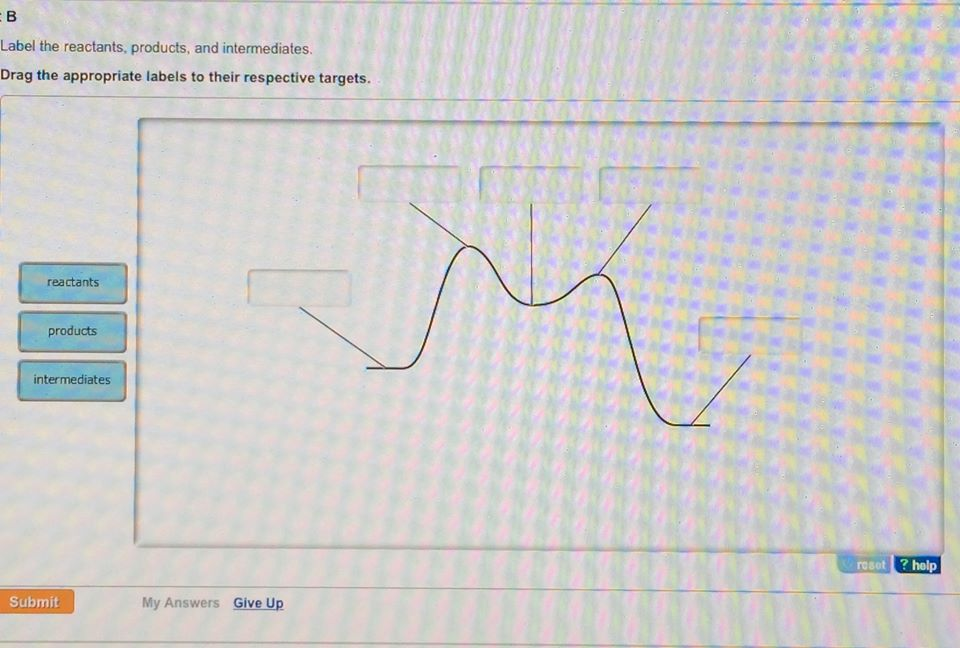
49+ label the energy diagram for a two step reaction
The intermediate then goes through a second step transition state 2 with the highest energy barrier to form the product. Energy required to break the bonds between atoms for the REVERSE reaction.
Solved Consider The Following Energy Diagram Showing The Chegg Com
Web See Answer Question.

. Determine the activation energy. Label the following multi-step reaction diagram Show transcribed image text Expert Answer 98 133 ratings Transcribed image text. Web Steps for Drawing the Reaction Energy Diagram of a Catalyzed Reaction.
Previous question Next question. Web The potential energy diagram shows an activation energy peak for each of the elementary steps of the reaction. Consider the following enthalpy or potential energy diagrams for a set of hypothetical chemical reactions.
Web Label the energy diagram for a two-step reaction. Label the reactants and the products and determine their energies Remember that reactants will be on the left and products on the right. The energy of the reactants is 1 kJmol and.
These diagrams show the potential energy of the reactants and products as well as the activation energy required for the reaction to occur. The valley between represents the intermediate for the reaction. Check Your Learning Reaction diagrams for a chemical process with and without a catalyst are shown below.
The energy profile can also be used to determine the overall change in energy for the reaction. An exothermic chemical reaction. Web Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step and relate this to the activation energy of the overall reaction.
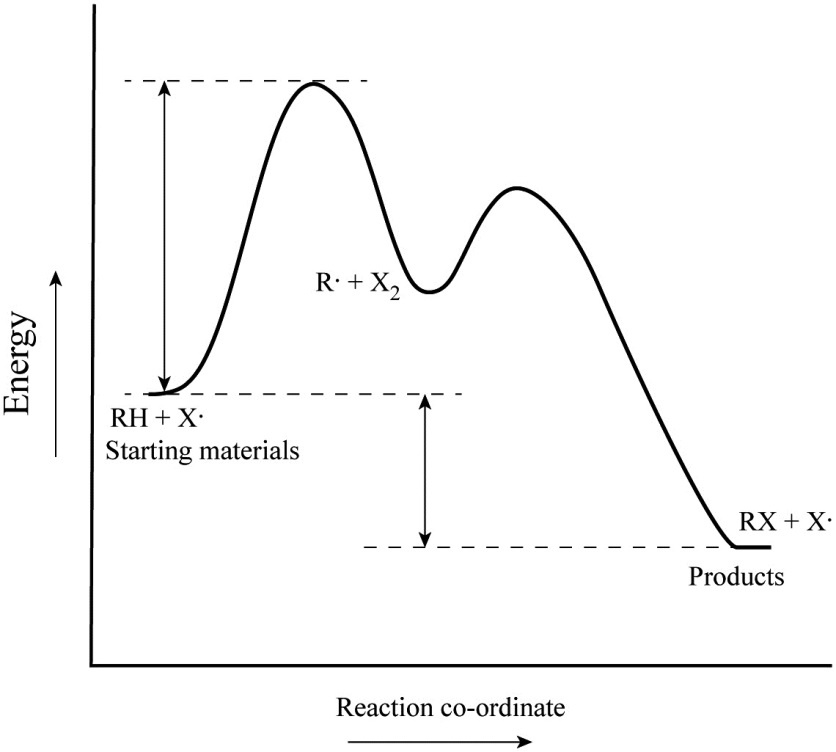
Web This is illustrated by the energy diagram where the activation energy for the first step is higher than that for the second step. Web Point of reaction in which all bonds between atoms are broken and atoms are free to recombine. Also recall that an S N 1 reaction has first order kinetics because the rate determining step involves one molecule splitting apart not two molecules colliding.
Enthalpy change transition state starting materials RXH products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved. Label the following multi-step reaction energy diagram. Both reactions involve a two-step mechanism with a rate-determining first step.
Write the rate law expression for a two-step mechanism in which the rate constants have significantly different magnitudes. Enthalpy diagram for an exothermic chemical. Figure2 The reaction whose potential energy diagram is shown in the figure is a.
In the above reaction a reactant goes through one elementary step with a lower activation energy transition state 1 to form the intermediate. Diagram in kinetics pt 2 folder in energy diagram folder on desktop 1- J 2- F 3- A 4- D 5- E 6- B 7- L 8- C 9- K -We can use a 2-D potential energy surface to. Web A physical or chemical process can be represented using an energy diagram which shows how the potential energy of the initial state relates to the potential energy of the final state.
Maximum amount of energy required to perform the reaction. Web A potential energy diagram is a graph with enthalpy potential energy on the vertical axis and the reaction pathway time on the horizontal axis. Determine the energies of the reactants and products and the reaction enthalpy Step 2.
Web The catalyzed reaction is the one with lesser activation energy in this case represented by diagram b. Web Label the following reaction coordinate diagram by matching between letters and numbers. Web The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step.
Label the following multi-step reaction energy diagram. Web Energy diagrams also known as potential energy diagrams can be used to represent the energy changes that occur during a chemical reaction. If the initial state has a lower potential energy than the final state the process is endothermic.
Web The energy diagram of a two-step reaction is shown below. Youll get a detailed solution from a subject matter expert that helps you learn core concepts.
Solved Consider The Following Energy Diagram Showing The Chegg Com
Label The Energy Diagram For A Two Step Reaction Home Work Help Learn Cbse Forum

Solved Based On The Reaction Coordinate Diagram Below Where Chegg Com

Energy Profile Chemistry Wikipedia
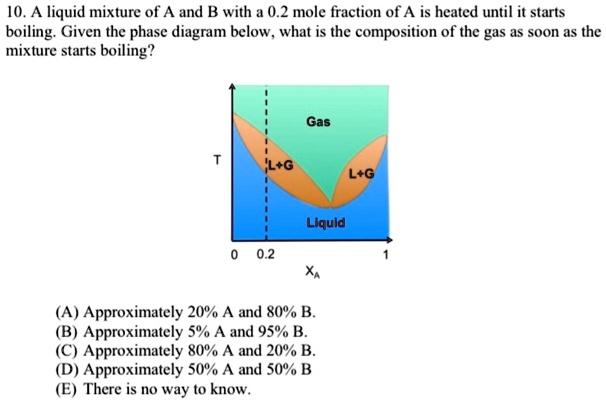
Browse Questions For Chemistry 101

Browse Questions For Chemistry 101

High Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry With Increased Throughput For Biomolecular Analysis Analytical Chemistry

Isotopic Resolution Of Protein Complexes Up To 466 Kda Using Individual Ion Mass Spectrometry Analytical Chemistry

Which Of The Following Energy Profile Diagram For A Three Step Reaction In Which The First Step Is Slowest And The Last Step Is Fastest Assume That Reaction Is Exothermic

Oneclass Label The Energy Diagram For A Two Step Reaction

Controlled Au Polymer Nanostructures For Multiphoton Imaging Prodrug Delivery And Chemo Photothermal Therapy Platforms Request Pdf

Pdf Resonance Asymmetry And External Field Effects In The Photorecombination Of Ti 4
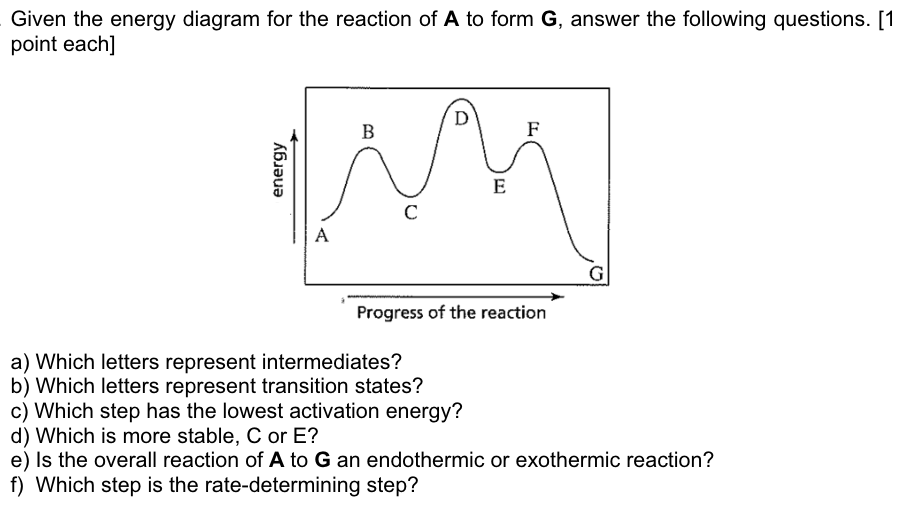
Solved Given The Energy Diagram For The Reaction Of A To Chegg Com
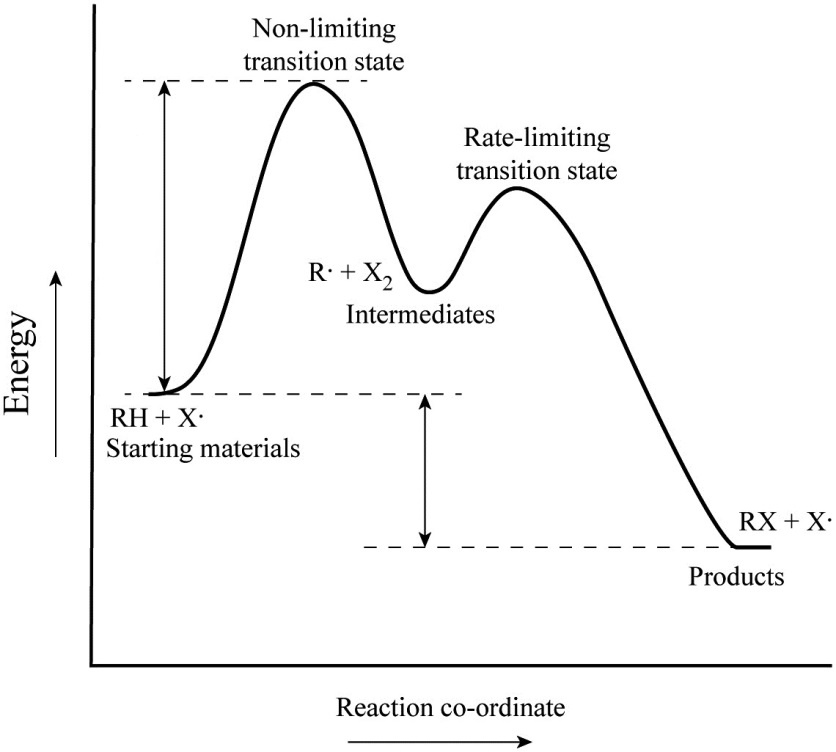
Label The Energy Diagram For A Two Step Reaction Home Work Help Learn Cbse Forum
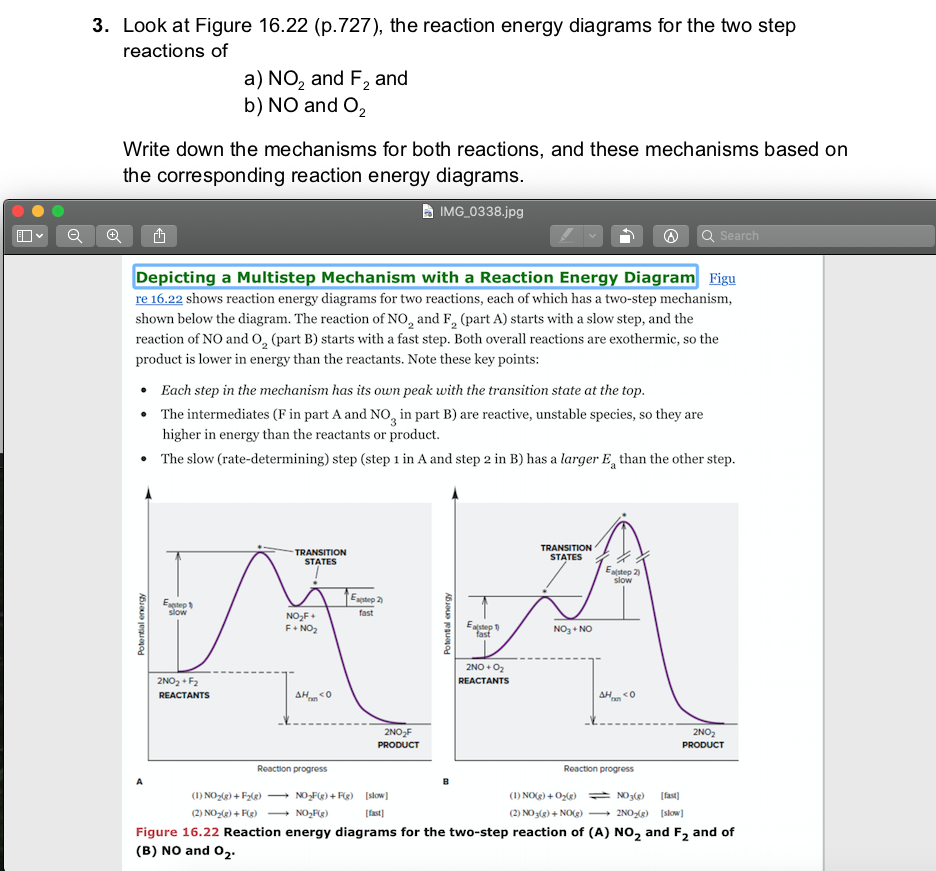
Solved 3 Look At Figure 16 22 P 727 The Reaction Energy Chegg Com
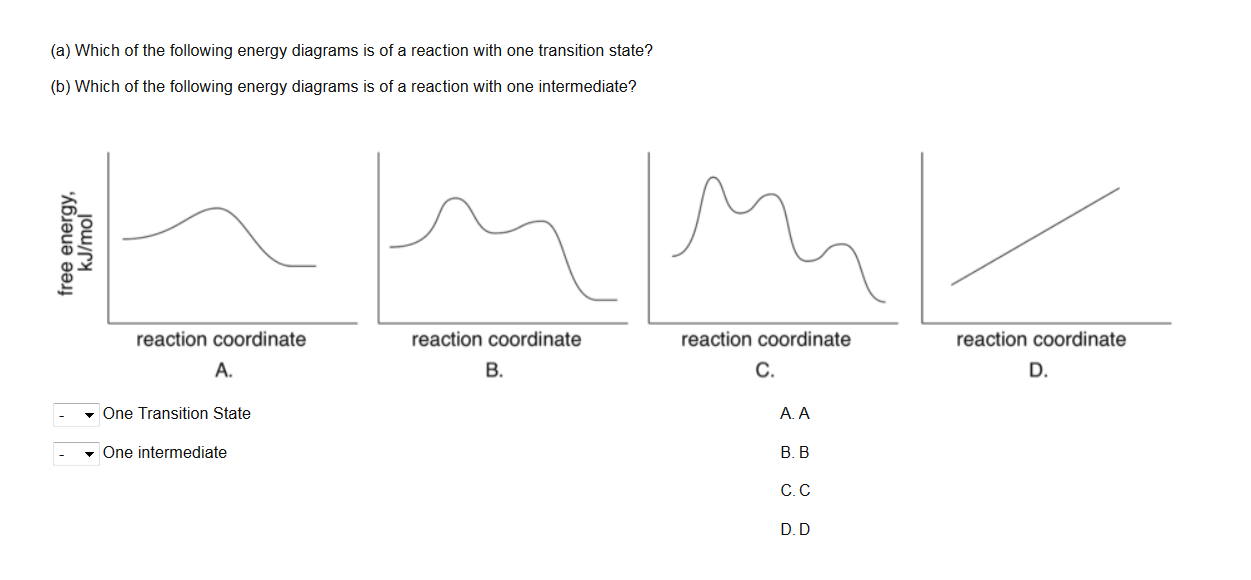
Solved Which Of The Following Energy Diagrams Is Of A Chegg Com
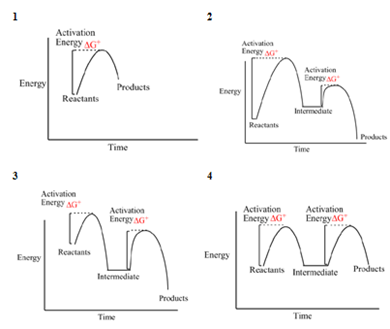
Solved Which Of The Following Energy Diagrams 1 2 3 Or 4 Chegg Com